The Challenge of Crystallization: A Persistent Barrier in Structural Studies
The challenge of protein crystallization represents one of the most unpredictable and demanding stages in structural biology research. Despite significant advancements in high-throughput screening techniques, many proteins—especially those that are membrane-bound, part of flexible complexes, or intrinsically disordered—continue to resist crystallization efforts.
This persistent barrier can lead to considerable delays in project timelines and can severely hinder drug discovery programs. When proteins fail to produce diffraction-quality crystals, researchers are faced with a critical decision: whether to continue with optimization efforts or shift their focus to alternative methods. NMR Spectroscopy emerges as a validated and effective solution, providing vital structural and dynamic information without the need for crystallization. This adaptability makes NMR a crucial method in the toolkit of structural biology.
NMR Spectroscopy: A Crystallization-Free Path to Structural Insights
NMR spectroscopy enables detailed structural analysis of proteins in the solution state, circumventing crystallization bottlenecks while providing unique access to protein dynamics.
Utilizing NMR Spectroscopy allows scientists to gain crucial insights into molecular movements and interactions that are often lost in crystallization, reinforcing the importance of NMR Spectroscopy in overcoming these challenges.
Key capabilities include:
- Analysis under near-physiological conditions
- Characterization of flexible regions and transient states
- Study of molecular interactions on relevant time scales
- Atomic-level information without crystal packing effects
For crystallization-resistant targets, NMR provides:
- Enables complete structure determinationfor many proteins up to ~30-40 kDa, and up to ~50 kDa with advanced NMR techniques
- Binding interface mapping and interaction surface analysis
- Quantitative dynamics analysis across multiple time scales
- Direct monitoring of conformational changes
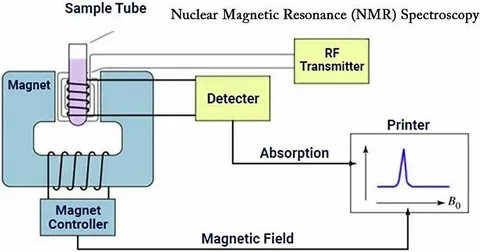
Identifying Ideal NMR Applications: Matching Technology to Research Needs
NMR demonstrates particular strength in several scenarios where crystallography faces limitations:
Membrane Protein Characterization
- Solid-state NMRenables structural studies of membrane proteins in native-like lipid environments, helping overcome poor solubility in aqueous buffers
- Enables study of lipid-embedded domains
- Provides insights into membrane protein dynamics
Intrinsically Disordered Proteins
- Captures transient structural features
- Maps binding-induced folding events
- Characterizes conformational ensembles
Drug Discovery Applications
- Identifies binding sites through chemical shift perturbations
- Quantifies weak interactions (equilibrium dissociation constants in the micromolar to millimolar range)
- Supports fragment-based drug discovery via saturation transfer difference NMR
Dynamic Process Analysis
- Monitors conformational changes
- Characterizes folding pathways
- Studies allosteric regulation mechanisms
Strategic Implementation: Integrating NMR into Your Research Pipeline
Technical Considerations:
- Sample Requirements: Soluble, stable proteins under experimental conditions, with molecular weights typically below 50 kDa for complete structure determination
- Isotope Labeling: Uniform 15N/13C labeling is typically required for backbone assignment, with additional 2H labeling for larger proteins
- Experimental Design: Method selection based on specific information requirements
Complementary Applications:
- Protein folding validation before crystallization trials
- Dynamics information to support crystallization strategies
- Alternative route for structurally challenging targets
Project Planning Factors:
- Timeline Considerations: Once expression and purification workflows are established, NMR studies can be initiated without the additional time required for crystallization screening and optimization
- Resource Allocation: Specialized instrumentation typically accessed through established facilities
- Data Integration: NMR-derived constraints enhance molecular modeling and support structure-based design
Platform Integration: Maximizing Research Outcomes Through Multi-Technique Approaches
Modern structural biology benefits from integrated strategies that combine complementary analytical techniques.
Integration Strategies:
- Sequential Methodology: NMR for initial construct screening and folding validation
- Parallel Data Collection: Combined crystallography and NMR for comprehensive analysis
- Data Validation: Cross-technique verification and model refinement
Platform Advantages:
- Risk Mitigation: Reduced dependency on single methodology
- Comprehensive Insights: Combined static and dynamic information
- Efficient Timelines: Parallel technical pathways prevent project stagnation
Conclusion
This integrated approach has proven particularly valuable for complex systems requiring both high-resolution structural data and dynamic behavioral information.
NMR spectroscopy offers a powerful alternative when crystallization presents formidable challenges, emerging as a vital complementary tool within the diverse strategies of structural biology. This technique is particularly advantageous for studying proteins that are difficult to crystallize, such as membrane proteins and intrinsically disordered proteins, which are essential for understanding biological processes.
The most successful structural studies leverage a combination of methodologies. They utilize the atomic-resolution precision offered by crystallography, allowing researchers to visualize the detailed arrangements of atoms within protein structures.
In tandem, NMR spectroscopy provides invaluable insights into solution-state dynamics, capturing information about protein interactions and conformational changes that occur in a native-like environment. Additionally, other specialized methods are employed to address specific research challenges, paving the way for a more thorough understanding of complex biological macromolecules.
This multi-technique perspective facilitates optimal methodology selection, enabling scientists to tailor their approach based on the unique characteristics of the protein and the specific objectives of their research. By integrating various analytical techniques, researchers can overcome technical limitations, thus expanding the potential for discoveries in structural biology.
Creative Biostructure plays a crucial role in supporting advanced structural biology research through integrated technology platforms. They offer expert guidance in both X-ray crystallography and NMR spectroscopy applications, ensuring that scientists are equipped with the tools and insights necessary to push the boundaries of knowledge in this rapidly evolving field.