Interstitial lung disease (ILD) represents a complex group of pulmonary disorders characterized by inflammation, fibrosis, and progressive loss of lung function.
For pharmaceutical and biotechnology companies developing next-generation respiratory therapeutics, understanding disease mechanisms at the site of pathology is essential.
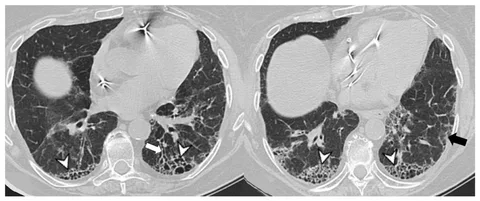
Among the most valuable biological resources enabling this insight are BAL samples from interstitial lung disease samples, which provide direct access to the pulmonary microenvironment.
As drug pipelines increasingly focus on mechanism-driven therapies, immunomodulation, and precision respiratory medicine, bronchoalveolar lavage derived bio-specimens are playing a critical role in accelerating discovery, validation, and clinical translation across multiple therapeutic areas.
Why BAL Samples Matter in Interstitial Lung Disease Research
Bronchoalveolar lavage is a minimally invasive clinical procedure that collects cellular and soluble components from the lower respiratory tract.
The resulting bronchoalveolar lavage fluid samples contain immune cells, cytokines, proteins, lipids, and nucleic acids that reflect ongoing pathological processes in the lung.
In ILD, where disease heterogeneity and overlapping phenotypes complicate diagnosis and treatment, BAL samples provide a disease-relevant snapshot that cannot be replicated by blood or tissue biopsies alone.
This localized insight is particularly valuable for pharmaceutical research programs aiming to:
-
Characterize inflammatory and fibrotic pathways
-
Identify novel drug targets
-
Validate biomarkers linked to disease progression or treatment response
-
Support translational and clinical research strategies
Supporting Drug Discovery Through Mechanistic Insight
One of the most significant advantages of using BAL samples from interstitial lung disease samples is their ability to reveal disease mechanisms directly within the lung microenvironment.
Cellular profiling of BAL fluid enables researchers to quantify macrophage activation, lymphocyte subsets, neutrophilic inflammation, and fibrotic signaling cascades.
For biotech companies working on antifibrotic agents, immune-targeted therapies, or regenerative approaches, BAL-based data helps refine target selection and reduce early-stage development risk.
These samples also support hypothesis testing in preclinical models by offering human-relevant validation before compounds move into costly clinical phases.
Accelerating Biomarker Discovery and Validation
Biomarker development remains a critical bottleneck in pulmonary drug development. ILD progression can be unpredictable, and traditional clinical endpoints often fail to capture early therapeutic effects.
Bronchoalveolar lavage fluid samples are increasingly used to discover and validate biomarkers that correlate with disease activity, prognosis, and drug response.
Proteomic, transcriptomic, and metabolomic analyses of BAL fluid allow pharma teams to identify candidate biomarkers that are biologically linked to lung pathology rather than systemic spillover effects.
These biomarkers can then be incorporated into clinical trial designs to improve patient stratification, monitor target engagement, and support regulatory discussions.
Enhancing Clinical Trial Design and Patient Stratification
Clinical trials in interstitial lung disease face challenges related to patient heterogeneity, slow disease progression, and variable treatment responses.
Integrating BAL samples into clinical research protocols offers a powerful tool for addressing these challenges.
BAL-derived biomarkers can help identify subpopulations more likely to respond to specific therapeutic mechanisms, enabling smarter trial enrollment strategies.
For example, distinguishing inflammatory-dominant versus fibrotic-dominant ILD phenotypes through BAL analysis can inform inclusion criteria and endpoint selection.
From a regulatory and clinical development perspective, this approach strengthens the biological rationale behind trial designs and supports more data-driven decision-making across development stages.
Expanding Applications Across Therapeutic Areas
While ILD is the primary focus, the value of BAL samples extends across multiple therapeutic areas relevant to pulmonary and systemic drug development. These include:
-
Fibrotic disorders, where BAL analysis helps track extracellular matrix remodeling and profibrotic signaling
-
Autoimmune and inflammatory diseases, particularly connective tissue disease–associated ILD
-
Infectious and post-infectious lung conditions, which share overlapping immune pathways with ILD
-
Oncology, especially in understanding tumor-associated inflammation and treatment-related pneumonitis
By leveraging BAL samples across these interconnected therapeutic areas, pharmaceutical companies can maximize the translational value of their respiratory research investments.
The Importance of Sample Quality and Standardization
For BAL samples to effectively support drug development, quality and consistency are non-negotiable. Variability in collection methods, processing timelines, and storage conditions can significantly impact downstream analyses and data reliability.
Pharma and biotech organizations increasingly partner with specialized bio-specimen providers and clinical research organizations that follow standardized protocols, ethical sourcing practices, and rigorous quality control measures.
High-quality BAL samples from interstitial lung disease samples ensure reproducibility, regulatory confidence, and long-term usability across discovery and clinical programs.
Integrating BAL Data with Multi-Omics and AI Platforms
Modern pulmonary drug development relies on data integration across multiple modalities. BAL samples are particularly well suited for multi-omics approaches, including single-cell sequencing, proteomics, and spatial biology.
When combined with advanced analytics, machine learning, and clinical datasets, BAL-derived insights help uncover complex disease networks and predict therapeutic outcomes more accurately.
This integration aligns with the broader industry shift toward data-driven drug development and systems-level understanding of lung disease.
Strengthening Translational Confidence in Pulmonary Pipelines
Ultimately, the strategic use of bronchoalveolar lavage fluid samples strengthens translational confidence by linking molecular findings to real-world disease biology.
For pharma and biotech companies navigating the high attrition rates of respiratory drug development, this connection is invaluable.
BAL samples reduce uncertainty, inform go/no-go decisions, and provide mechanistic evidence that resonates with internal stakeholders, investors, and regulatory bodies alike.
Conclusion
As interstitial lung disease continues to represent a significant unmet medical need, innovative research tools are essential for advancing effective therapies.
BAL samples from interstitial lung disease samples offer unparalleled access to the pulmonary microenvironment, enabling deeper biological insight, smarter clinical strategies, and more resilient drug pipelines.
For pharmaceutical and biotechnology companies focused on respiratory innovation, integrating high-quality BAL samples into research and development workflows is no longer optional—it is a strategic advantage that accelerates progress across discovery, clinical development, and multiple therapeutic areas.