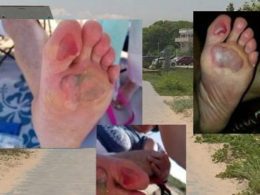
In a world grappling with the constant threat of infectious diseases, the emergence of groundbreaking vaccines offers hope and a potential solution to combat deadly pathogens. One such vaccine that has recently gained attention is Jynneos, a revolutionary immunization against Mpox. In this article, we delve into the intricacies of Jynneos, its development, and its role in enhancing immune protection against Mpox.
Unraveling the Threat of Mpox:
Mpox, a highly contagious and potentially lethal viral disease, has long plagued humankind. It spreads through close contact, respiratory droplets, and contaminated surfaces, posing a significant public health risk. The symptoms range from flu-like manifestations to severe complications, including encephalitis and hemorrhagic fever. The urgent need for an effective vaccine to counter this formidable adversary cannot be overstated.
The Rise of Jynneos:
Jynneos, developed by a team of dedicated scientists and researchers, stands as a promising response to the Mpox threat. It is a novel, non-replicating vaccine that combines two components: Modified Vaccinia Ankara (MVA) and Modified Vaccinia Istanbul (MVI). The unique combination of these viral vectors stimulates a robust immune response while maintaining a high level of safety.
Efficacy and Mechanism of Action:
Jynneos works by introducing weakened forms of the Mpox virus into the body, triggering a targeted immune response. The MVA component elicits a response from the T cells, which are responsible for the cellular defense against viral infections, while the MVI component stimulates the production of Mpox-specific antibodies. This dual mechanism enhances the immune system’s ability to recognize and neutralize the virus rapidly.
Clinical Trials and Safety Profile:
Extensive clinical trials have been conducted to assess the safety and efficacy of Jynneos. The vaccine has demonstrated remarkable results, showing a high rate of protection against Mpox infection. Moreover, Jynneos has shown no significant adverse effects in the majority of recipients, with only mild and transient side effects reported, such as injection site reactions or mild flu-like symptoms.
Global Impact and Future Prospects:
The availability of Jynneos opens up new avenues in the battle against Mpox, particularly in regions where the disease poses a severe public health burden. The World Health Organization and other global health entities are actively collaborating to ensure the equitable distribution and administration of Jynneos in affected areas. This effort aims to curtail the spread of Mpox and prevent outbreaks that can potentially escalate into global health crises.
Ethical Considerations and Public Perception:
As with any medical intervention, ethical considerations surrounding the use of Jynneos remain crucial. Transparent communication regarding its development, safety, and potential benefits is essential to foster public trust and acceptance. The involvement of diverse communities, robust regulatory oversight, and adherence to rigorous safety standards are vital to ensure the ethical implementation of Jynneos.
Conclusion:
The advent of Jynneos represents a significant breakthrough in the quest for enhanced immune protection against Mpox. This innovative vaccine has the potential to save countless lives and mitigate the devastating consequences of Mpox outbreaks. As ongoing research and global collaboration continue to refine and optimize its use, Jynneos stands as a testament to humanity’s unyielding commitment to combating infectious diseases and safeguarding public health.