What is thermodynamics?
Thermodynamics is the branch of physics that deals with the relationships between heat and other forms of energy. It studies how energy is transferred between different forms and how it affects the properties of matter.
Importance of thermodynamics in various fields
Thermodynamics plays a crucial role in various fields such as engineering, chemistry, biology, and environmental science. It provides the fundamental principles for understanding and analyzing complex systems.
The Laws of Thermodynamics
First Law: Conservation of Energy
The first law of thermodynamics states that energy cannot be created or destroyed, only transformed from one form to another. This law is also known as the law of conservation of energy.
Second Law: Entropy and the direction of processes
The second law of thermodynamics introduces the concept of entropy, which measures the degree of disorder or randomness in a system. It states that the entropy of an isolated system tends to increase over time, leading to the directionality of processes.
Third Law: Absolute zero and the unattainability of it
The third law of thermodynamics states that as the temperature of a system approaches absolute zero, the entropy of the system approaches a minimum value. Absolute zero is the lowest possible temperature and represents the point at which the entropy of a perfect crystal is zero.
Thermodynamic Systems
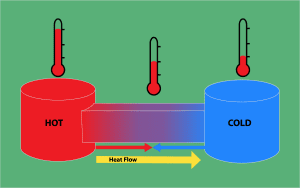
Definition of a system
In thermodynamics, a system is a specific region of space containing matter and energy, which is being studied.
Types of thermodynamic systems
There are three main types of thermodynamic systems:
- Closed system: A system that can exchange energy but not matter with its surroundings.
- Open system: A system that can exchange both energy and matter with its surroundings.
- Isolated system: A system that cannot exchange energy or matter with its surroundings.
Thermodynamic Processes
Definition of a process
A thermodynamic process is any change that occurs in the state of a system.
Types of processes
There are several types of thermodynamic processes, including:
- Isothermal process: A process that occurs at constant temperature.
- Adiabatic process: A process that occurs without the transfer of heat between the system and its surroundings.
- Isobaric process: A process that occurs at constant pressure.
- Isochoric process: A process that occurs at constant volume.
Thermodynamic Variables
Thermodynamics deals with several key variables, including:
- Temperature: A measure of the average kinetic energy of the particles in a system.
- Pressure: The force exerted by a substance per unit area.
- Volume: The amount of space occupied by a substance.
- Internal energy: The total energy contained within a system.
- Enthalpy: The sum of the internal energy and the product of pressure and volume.
- Entropy: A measure of the disorder or randomness in a system.
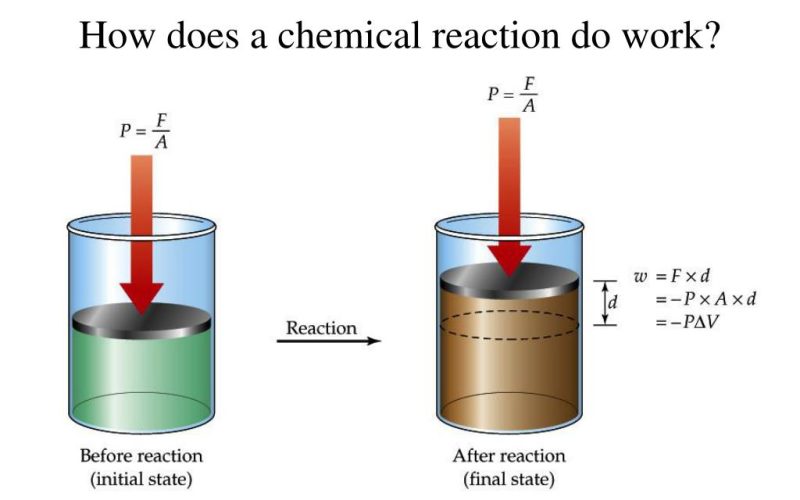
Heat and Work
Definitions of heat and work in thermodynamics
Heat is the transfer of energy between a system and its surroundings due to a temperature difference. Work is the transfer of energy that results in a change in the state of a system.
Sign conventions for heat and work
In thermodynamics, heat and work are often expressed with specific sign conventions. Heat added to a system and work done by a system are considered positive, while heat removed from a system and work done on a system are considered negative.
Applications of Thermodynamics
Steam engines and power plants
Thermodynamics has been instrumental in the development of steam engines and power plants, which harness energy from heat to perform mechanical work.
Refrigerators and air conditioners
Refrigerators and air conditioners operate based on thermodynamic principles, utilizing the transfer of heat to achieve cooling effects.
Chemical reactions and equilibrium
Thermodynamics plays a crucial role in understanding chemical reactions and predicting the conditions under which they occur. It also provides insights into chemical equilibrium and reaction spontaneity.
Thermodynamics in Everyday Life
Cooking
Cooking involves various thermodynamic processes such as heating, cooling, and phase changes, making thermodynamics essential for understanding the science behind cooking techniques.
Automobile engines
The operation of automobile engines relies on thermodynamic principles, where the combustion of fuel generates heat to produce mechanical work.
Electronics
The design and operation of electronic devices, such as computers and smartphones, incorporate thermodynamic principles to manage heat dissipation and energy efficiency.
Challenges and Future of Thermodynamics
Advancements in nanotechnology have brought new challenges and opportunities for thermodynamics, as researchers explore the behavior of materials at the nanoscale and develop innovative technologies.
Renewable energy and thermodynamics
The transition to renewable energy sources like solar and wind power involves complex thermodynamic processes for energy conversion and storage, driving research into more efficient and sustainable energy systems.
Conclusion
Thermodynamics is a fascinating field that underpins our understanding of energy and its interactions with matter. From the laws that govern energy conservation to the practical applications in everyday life, plays a vital role in shaping modern science and technology.
FAQ
What is the importance of thermodynamics in engineering?
Thermodynamics provides the foundational principles for understanding energy transfer and conversion, which are essential for the design and operation of engineering systems.