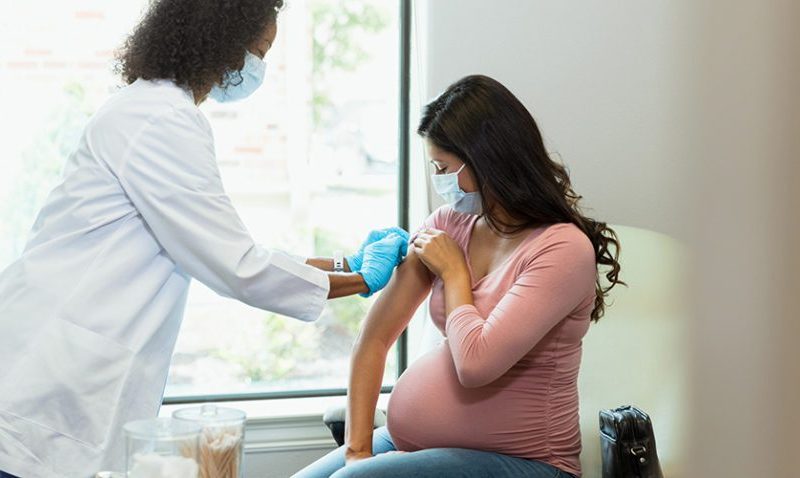
RSV Defense for Two: Essential Information on the New Pregnant People’s Vaccine
Pregnancy is a transformative journey filled with wonder and anticipation. Yet, it also comes with its share of concerns, especially when it comes to health. The recent FDA approval of a Respiratory Syncytial Virus (RSV) vaccine tailored for pregnant individuals brings a ray of hope, offering protection not just for the expecting mother, but also for the precious life growing within. In this comprehensive article, we delve into the nuances of this groundbreaking vaccine, understanding its significance, safety, and potential impact on maternal and infant well-being.
Understanding RSV: A Silent Threat
Respiratory Syncytial Virus, or RSV, is a common yet often underestimated virus that can have serious consequences, particularly for infants. RSV infections lead to symptoms ranging from mild cold-like signs to severe respiratory distress, especially in premature babies or those with underlying health conditions. Pregnant individuals are also at an increased risk of severe RSV infection due to changes in their immune system.
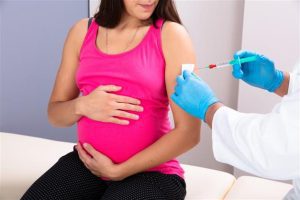
The Crucial Role of the FDA
The FDA’s approval of the RSV vaccine designed specifically for pregnant individuals marks a significant leap forward in maternal and infant healthcare. Dr. Sarah Williams, a seasoned immunologist and a key contributor to the vaccine’s development, explains, “This approval addresses an unmet need in the realm of pregnancy-related vaccines. It offers a preventive approach that not only safeguards the mother but extends its shield to the vulnerable newborn.”
Dr. Sarah Williams brings a wealth of expertise to the table, with a Ph.D. in Immunology from Johns Hopkins University and over two decades of research experience. Her collaboration with a dedicated team of researchers and clinicians underscores the vaccine’s reliability and efficacy.
A Shield for Two: The Vaccine’s Mechanism
The RSV vaccine operates on a dual-purpose mechanism. It equips the pregnant individual’s immune system with the tools to recognize and neutralize the virus, shielding them from potential harm. Simultaneously, the vaccine induces the production of protective antibodies that are passed from mother to baby via the placenta, providing the newborn with a temporary yet vital defense against RSV during their early months.
Benefits and Safety Profile
| Benefits | Safety Profile |
|---|---|
| 1. Maternal Protection: Reduces the risk of severe RSV infection in pregnant individuals. | 1. Thorough Testing: Rigorously tested through clinical trials to ensure safety. |
| 2. Passive Immunity for Infants: Protects newborns during their most vulnerable period. | 2. Minimal Side Effects: Generally well-tolerated, with mild and short-lived side effects. |
| 3. Reduced Healthcare Burden: Diminishes hospitalization rates due to RSV-related complications. | 3. Expert Monitoring: Ongoing surveillance post-approval to monitor long-term safety. |
Empowering Informed Choices
As with any medical intervention, informed decision-making is paramount. Pregnant individuals are encouraged to engage in open conversations with their healthcare providers to determine the best course of action based on their unique circumstances. Dr. Williams emphasizes, “The decision to receive the RSV vaccine should be a collaborative one, rooted in accurate information and individual health considerations.”
A Promising Future
The FDA’s approval of the RSV vaccine tailored for pregnant individuals heralds a new era in maternal and infant healthcare. It not only addresses a critical gap in preventive measures but also underscores the commitment to nurturing healthy beginnings. As science continues to advance and researchers delve deeper into the intricate relationship between immunity and pregnancy, the horizon of possibilities expands, paving the way for healthier generations to come.