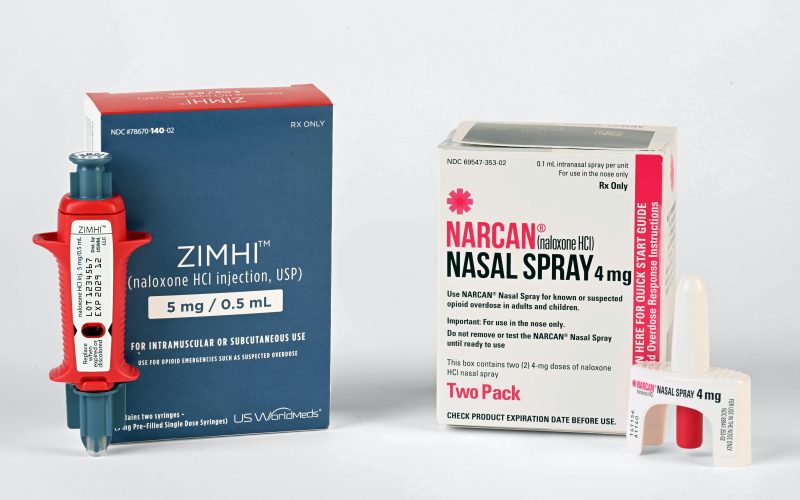
The U.S. Food and Drug Administration (FDA) has made a significant move in the fight against the opioid crisis by granting over the counter (OTC) status to Narcan, a medication used to reverse opioid overdoses. This decision is expected to widen availability and accessibility of this life-saving medication, potentially saving more lives in the ongoing battle against opioid overdose deaths.
Body: The opioid crisis has been a pressing public health issue in the United States for years, with staggering rates of overdose deaths. As the crisis continues to escalate, finding effective solutions to prevent opioid overdose deaths has become a top priority for policymakers, public health officials, and communities across the nation.
In response to this urgent need, the FDA has granted OTC status to Narcan, a brand name for naloxone, a medication that quickly reverses the effects of opioid overdose. Previously, Narcan was only available with a prescription from a healthcare professional, which could create a barrier to timely access in emergency situations. The decision to grant OTC status to Narcan aims to remove this barrier and make the medication more readily available to those who may need it to save a life.
Advocates of the FDA’s decision applaud the move, citing that increased availability of Narcan can empower individuals, families, and communities to take action in preventing opioid overdose deaths. With OTC status, Narcan can be obtained without a prescription from pharmacies or other authorized retailers, making it more accessible in emergency situations where time is of the essence.
However, there are also concerns and criticisms surrounding the decision. Some express worries about potential misuse of Narcan, inadequate training in administering the medication, and the need for comprehensive addiction treatment services to accompany expanded access to the medication. Critics also highlight that OTC Narcan may not address the root causes of the opioid crisis, such as addiction and the availability of opioids in the market.
To address these concerns, the FDA’s decision to grant OTC status to Narcan was based on a thorough review of available evidence, including safety and efficacy data. The FDA considered the potential benefits of increased accessibility in saving lives, as well as the risks of potential misuse, and determined that the benefits outweighed the risks.
Public health organizations, such as the Centers for Disease Control and Prevention (CDC) and the Substance Abuse and Mental Health Services Administration (SAMHSA), have long advocated for expanded access to Narcan as a critical tool in preventing opioid overdose deaths. The FDA’s decision aligns with these recommendations and reflects a proactive approach in addressing the opioid crisis.
In conclusion, the FDA’s recent decision to grant over-the-counter status to Narcan is a significant step in widening availability and accessibility of a life-saving medication for opioid overdose reversal. While the move has been met with both support and skepticism, it reflects a proactive approach in addressing the opioid crisis and saving lives. As the situation continues to evolve, it will be essential to monitor the impact of OTC Narcan on reducing opioid overdose deaths and ensuring appropriate use of the medication.